Microplastics Are Secretly Breeding Superbugs – Here’s How
I’ve been reading a lot about microplastics recently, both out of personal concern and because I’m thinking about focusing on this for my PhD. One paper (Zhou et al., 2024) that really stood out examined how microplastics help bacteria share antibiotic resistance genes. What made it special was how thorough their methods were.
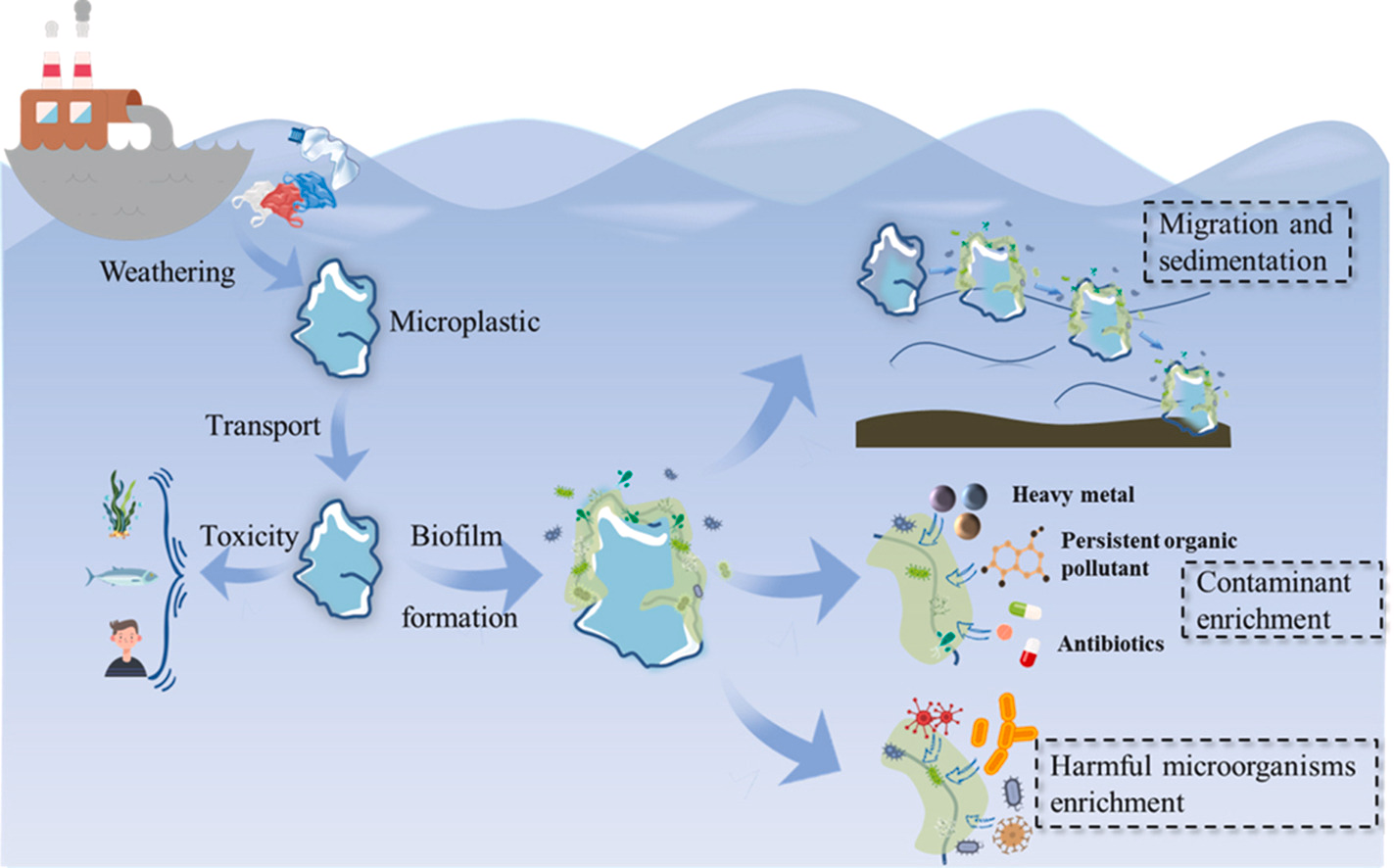
The researchers chose two specific types of E. coli bacteria for their experiments. One strain carried antibiotic resistance genes on a special plasmid, while another strain had different resistance markers. This smart pairing made it easy to track when genes moved between them. They tested four common microplastic types found in rivers and coasts, all cut to the same exact size for consistency. Instead of using plain water, they created artificial estuary conditions that matched real world environments where this gene swapping might happen. This attention to detail was important because factors like salt levels and available nutrients can change how bacteria behave.
Their approach to measuring gene transfer was particularly clever. After letting bacteria grow on the microplastics for several days, they carefully removed the bacterial films and spread them on special plates containing antibiotics. Only bacteria that had successfully acquired new resistance genes could grow on these plates. By counting these survivors, they could calculate exactly how often genes were being transferred. One of their best decisions was including a control group using bacteria that couldn’t physically connect to share genes. This proved the gene transfer required direct bacterial contact rather than just free floating DNA. It’s this kind of careful experimental design that makes their results so convincing.
When they looked at the molecular details, things got even more fascinating. They found that bacteria on microplastics activated more genes for surface proteins and the tiny tubes they use to share DNA. At the same time, stress markers didn’t change much, showing it was the biofilm environment itself causing the increased gene swapping, not some general stress response. They also tested different growth conditions and found that adding glucose boosted gene transfer, probably by giving bacteria more energy. The process peaked around three days in, which matches how long it takes for biofilms to fully develop. What makes this study impressive is how completely it examines the problem. It doesn’t just show that gene transfer happens on microplastics, but carefully explains why and how. As someone considering research in this area, it’s both inspiring and a bit intimidating to see how much work goes into properly understanding these complex environmental interactions. This paper clearly shows that microplastics don’t just pollute our waters passively, but actively change microbial ecosystems in ways we’re only starting to grasp.
(Also, I now have a mild fear of polyethylene. Thanks, science.)
References
- Mar. Environ. Res.Microplastic biofilms promote the horizontal transfer of antibiotic resistance genes in estuarine environmentsMarine Environmental Research, Nov 2024
- J. Hazard. Mater.Biofilm on microplastics in aqueous environment: Physicochemical properties and environmental implicationsJournal of Hazardous Materials, Feb 2022
Enjoy Reading This Article?
Here are some more articles you might like to read next: